Radio waves in MRI
Author : Ms. Kalpana Parajuli
MSc Medical Imaging Graduate
It is true that we, who are associated with field of medical
imaging, are mainly concerned with detrimental effects of ionizing
electromagnetic radiation. It seems that our attention is biased and it is high
time we realized we are surrounded by non-ionizing radiation and we should know
how they interact with biological tissue. This article will mainly focus on radio
waves, electromagnetic waves of frequency ranging from 9 kHz and 300 GHz
that are used to perturb the longitudinal magnetization in order to
produce MR signal. Those of us who belong to this dynamic and ever progressing
field of medical imaging should know that the way by which radio wave interacts
with the matter is quite different from the pathway that ionizing radiation
such as x radiation takes. It is because these waves lack enough energy needed
for compton or photoelectric interaction. For the ionization or the breakage of
covalent bond to occur a single photon should interact with the electrons in
the atomic orbital which is not possible with RF waves that releases its energy
through the interaction of multiple photons. The effects of RF energy in human
body can be divided into two categories.
a) Thermal also called dielectric heading:
Heating effect of RF
waves is best explained by the phenomenon polar molecules show under an electri
field. Radio waves are nothing but the oscillating electric and magnetic
fields. Under the sinusoidally changing electric field, the polar molecules,
molecule with non-zero dipole moment, begin to rotate, as a result of which
collision between molecules occurs followed by energy transfer from these
molecules to the adjacent molecules. The resultant agitation and energy
transfer cause increase in temperature and production of heat respectively. Similarly
changing magnetic field induces the electric field (Faradays law) in human body
that deposits the energy in the form of heat as described above.
The behavior of electric and magnetic field of radio waves depends
on the distance between the source of radiation and the object on which it is
incident, as well as on its frequency. The patient in MRI is exposed to waves
of frequency that range from 8.5 to 340 MHz and the scanner is in the “near
field” i.e “d” is less than one wavelength so heat production is mostly by the
magnetic field of RF waves with little or minimal effect of electric field of
RF wave. The reason behind this sort of dominance is the independent
relationship between “E” and “B” in the near field.
b) Non-Thermal :
These are specific effects that occur due to interaction of
magnetic and electric field vector with human body other than heating. Very
less is known about non-thermal effects because of lack of conclusive
scientific evidence in human models and therefore not taken into account by
regulatory bodies while providing guidelines on safety limit.
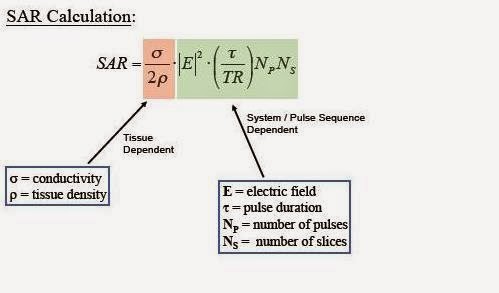
Specific Absorption Rate (SAR) is the name of dosimetric
quantity used to describe the rate of heat deposition in unit mass of tissue,
and is measured in W/kg.
What affects SAR in MR
imaging?
The answer to this question is given by the figure below.
It is thus clear from above formula that SAR is the function
of patient related factors as well as scan parameters. SAR increases with the
square of magnetic field strength of the magnet system of scanner, because high
power radio waves are needed to cause resonating effect on the spins of higher
larmor frequency. It also increases with the square of the flip
angle, whereas it varies proportionally with the duty cycle (ratio of average
to peak RF power) and patient weight. Because conductivity and tissue density
vary from person to person, the calculation of SAR is not easy and accurate.
Animal experiments have shown that the permittivity and conductivity of tissue
decreases with age thus young ones are more vulnerable to deleterious effects
of radio waves. It also depends on the perfusion status and geometric
configuration of exposed tissue and presence of metallic implants. Dependency of
SAR on several factors is one reason behind the challenges in RF dosimetry.
SAR of 1W/kg
is said to cause increase in temperature of insulated object (phantom) by 1⁰C
in an hour. In human and animals, input of 4W/kg of SAR has shown to raise the
temperature by 1⁰C. It is not practical to measure change in temperature
(core/whole body or localized) thus SAR is used to quantify the RF exposure. International
Electrotechnical Commission( IEC 60601-2-33) and Food And Drug Administration (FDA) has
given limits for both temperature as well as values of SAR (shown below).
The guidelines consist of limits for the whole body and local level exposure,
because some organs are highly heat sensitive than others because of higher
resistance, and are less affected by thermoregulatory system of body because of
less perfusion, for example: eye, gonads,
and thus separate limit for local RF exposure was required.
It is now realized that separate guideline is
necessary to account the change (increase) in SAR by metal implanted in the
body of patients. However none of the current guidelines have addressed this
issue. The limits are also given for occupational exposure. Those given by (IEC) are same as that for patients
whereas that by Institute
of Electrical and Electronics
Engineers (IEEE)
and International Commission on Non-Ionizing Radiation (ICNIRP) are one tenth
of the maximum limit for patients.
Just like CTDI volume and Dose Length Product
are displayed in the CT scanner, SAR value is also displayed on the monitor of
the MRI system. There are many methods available for estimation of SAR. Two
basic methods are caloriemetric method and pulse energy method. At the
beginning of the scanning the machine runs calibration to find out the energy
required to flip the spins by 90 and 180 degree. Power is then obtained by
dividing the total energy of all pulse in one sequence with time of repetition
(TR), and the result is ultimately divided by weight of the patient to get SAR.
This is why the scanner requires us to input the value of weight of the
patient.
Till now we
believe that increase in patient weight increases the SAR and all of us have
habit of looking at the value of SAR given by the machine itself to determine
whether our protocols are safe. However results of a recent study were quite
astounding because negative correlation was observed between patient weight and
the SAR calculated by 3T scanner, whereas in 1.5 T scanners the relationship
was maintained. This study has indeed raised a big question mark on the
reliability of the values of SAR provided by the manufacturers of MRI scanner.
What about the consequences of RF
induced heating in human?
Many incidents of second and higher degree burn in patients undergoing
examination in 1.5 and 3.0T MR systems have been reported. While documentation
of adverse consequence of RF associated heating of the metallic implants are
available, other physiologic response for example- changes in heart rate, oxygen
saturation, blood pressure, respiratory rate and cutaneous blood flow has not shown to cause effects that needs serious
concern.
What are the possible ways to reduce
SAR in MRI?
We are familiar with the tradeoffs between the radiation dose in CT and
the image quality. A balance between them is essential part of protocol
selection. Similarly in MRI as well, SAR can be minimized through wise
selection of parameters, which however also affects other areas like imaging
time, image quality etc. For example: reducing the flip angle affects the image
contrast, reducing the no of slices increases imaging time. Other methods can
be; reducing the echo train length of turbo spin echo or fast spin echoes, the use of
quadrature rather than linear coils for
transmission. Parallel imaging technique, by using multiple
receivers increases the amount of data received and thus reduces the imaging
time by a certain factor (reduction factor) without the use of additional RF
pulse and thus reduces SAR.
In conclusion safety issues regarding use of radio waves in MRI
are gaining much more attention than ever mainly because of three reasons - 3T
scanners, that needs high power RF amplifiers, about 35 kW, are widely being
used; the popularity of new RF intensive sequences (HASTE, FIESTA, true FISP) have
also heightened and MRI is no longer contraindicated for the patients with
implanted materials. Thus it is necessary that manufacturer, the scientific
research community and user should collaborate with each other to prevent the
potential hazardous situation that can arise due to RF energy in MR
environment.
MSc Medical Imaging Graduate Ms. Kalpana Parajuli
Maharajgunj Medical Campus (MMC),
Tribhuvan University Teaching Hospital (TUTH),
Kathmandu, Nepal